Electrolysis of water offers an effective and environmentally friendly solution for the production of hydrogen, decentralized, and depending on needs, what others can not see the gas. If the electricity will come from renewable energy sources, it makes the resulting gas environmentally friendly "green hydrogen".
All cells use two electrodes, oxygen is produced at one and hydrogen at the other. To split oxygen and hydrogen gases, required some kind of membrane or diaphragm. These membranes or diaphragm creates a high flow resistance and thus increase the time and cost of hydrogen production.
for many years, many scientists have tried to use a membraneless electrolysis, because it opens the way to reduce cost. Removal of membrane and associated auxiliary components can not only reduce capital costs but increase operational flexibility. Membrane or diaphragm have a limited operating temperature. Exceeding this set value affects the service life of the electrolytic cell and the purity of the gas that it produces. Operation without membrane eliminates those limitations, creating the possibility to work in more efficient conditions such as high engine temperature.
Platinum provides exponential performance improvements of the electrolyzers used for the production of green hydrogen, says CEO of Hydrox Holdings Corrie de Jager, who describes the efficiency of platinum as a "remarkable rise".
"We tried to add to the alloy with only 9% of platinum, and it gave a huge improvement of the electrolytic properties of a system," says de Jager. "Suddenly you get a much higher current density when using the platinum that you need. Small unit with high current density".
"You can build a smaller system and reduce costs. As for the operation, then the closer can be your electrodes, the higher the efficiency and the less energy they will consume. It's amazing how platinum suddenly increases efficiency. It's just "wow". It's stunning," added de Jager.
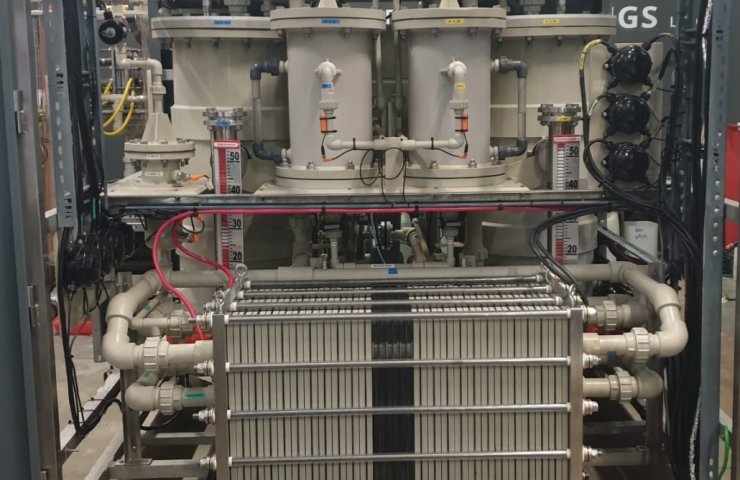
the Company Hydrox, sponsored by the Energy company Shell, has built a hydrogen cell in his lab in Strigampire, Randburg, Johannesburg, using their own divergent electrode flow (DEFT ™), the technology that allows the cells to operate without membranes at higher temperatures. This leads to a significant increase in the electrical efficiency and promises to reduce the cost of pure hydrogen, demand for which is growing by leaps and bounds around the world.
In the early experiments, the Nickel and not platinum was used, the main electrode metal: "We really couldn't afford platinum. I have to say that when we carried out tests with platinum, we had to send our electrodes to America, and the guys paid us platinum and sent it back."
"But in a short time we received the reports that were just spectacular. We received 1 100 milliamps per square centimeter ... Platinum, obviously, is expensive, so you should consider that you can use, and there are other metals associated with platinum, which really can reach platinum. Molybdenum is one of them, and there are a few others not expensive, but they can all play a role. Iron may play a role."
"We know how to do it, and we know how to ensure its durability and how to attach it to our electrodes. In addition, we will now consider different types of electrodes, a larger surface area. It will be stunning, and then we will have unmatched performance," says de Jager.
the electrochemically Electrolysis splits the water into hydrogen and oxygen, and for more than a century, the electrolyzers were limited to low temperatures and pressures, wherein the electrolysis is mainly carried out using some form of membrane for gas separation.
Now the world has a new local system, which was achieved thanks to the financial support of the program Gamechanger oil giant Shell, which provides businesses with start-up funding to promote unproven ideas in the early stages, which can improve the energy future.
Support Shell is vital for the company Hydrox, which is a National award in the field of scientific and technical forum in South Africa for innovation.
Standard cells using the system of the heat exchanger to remove excess heat that it does not exceed the maximum operating temperature of the membrane. But with DEFT ™ this excess temperature can be "locked" in the system to improve system efficiency, which reduces operating costs and the cost of hydrogen. The new system also can withstand fluctuations of current, making it ideal for the use of renewable energy and "green" hydrogen.